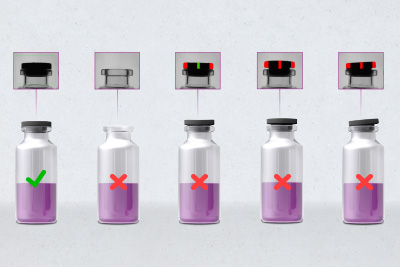
Vial inspection: Identify closure defects and product splashing!
An intelligent optical recognition unit from HEUFT, which will be presented at PharmaTechnica 2026 at stand A37, safeguards the container closure integrity (CCI) of filled vials. To do this, it checks the presence and correct seating of their rubber stoppers, while also identifying unwanted product splashing of freeze-dried preparations. Its hygienically optimised design allows it to be used in laminar flow areas of class A clean rooms.
There is no getting around it: parenteral drugs must be manufactured and filled under clean room conditions. This is the only way to ensure a defined environment with sterile, particle-free air for microbially safe end products. The vials in which they are packed must not leave this hygienically more demanding area until they are completely and hermetically sealed. If it is only later discovered that they are not sealed, it is already too late: there is a risk of dangerous recontamination, and the complete drug safety is at stake.

A similarly serious threat to the integrity and sterility of the vial closure area is also posed by what is known as splashing or fogging of lyophilised, i.e. freeze-dried, preparations, which are now used to fill a good 50 percent of all injection bottles. Depending on the system configuration, these undesirable quality defects occur during high-speed filling due to the increased flow rate of the product in the vial. Among other things, this can result in damage to the structure of the lyo cake and product residues in the form of unsightly spots and streaks on the stopper and in the neck area of the primary packaging material. The latter in particular can certainly pose a critical quality problem.
Defect detection and rejection in a laminar flow atmosphere
Primary packaging materials affected by such contamination and closure defects should therefore ideally be identified and rejected during the filling and closing process in a turbulence-free laminar flow atmosphere. The HEUFT e-mono, a rejection system designed for hygienically sensitive environments, does this. It is not pneumatically driven but by a particularly developed electric motor and thus does not emit any exhaust air.
A compact detection unit for optical stopper position checks and product-splashing detection based on a highly automated device platform for different quality check and in-line inspection systems from a common modular system is responsible for the previous fault detection. A HEUFT reflexx A.I. sensor camera developed and manufactured in-house for this purpose is securely integrated into a casing with an IP protection class of 67 which has been particularly designed for use in a laminar flow environment.
Intelligent camera, special lighting scenario

The intelligent HEUFT reflexx A.I.camera itself has integrated adaptive LED lighting and its own hardware and software which processes and evaluates its high-resolution images in real-time directly on the spot. Classic image analysis methods are combined with the latest AI methods such as the independent detection, calculation, classification and teaching of good and bad objects in order to increase the accuracy of the detection and to minimise the false rejection rate, i.e. the proportion of correctly filled vials that are incorrectly rejected. And all this is done with pixel-perfect accuracy under a special lighting scenario: the top of the vials is scanned in transmitted light so that their contour is clearly visible in the images.
In this way, even before the crimp cap is put on, it is possible to see whether the rubber stopper is actually present and correctly seated. If it is missing, has not reached the intended end position or is tilted, the primary packaging concerned is evaluated as defective and possibly leaky by the intelligent image analysis. In this case, the cap can no longer be crimped correctly; dangerous gaps appear, endangering the microbial purity of the sensitive pharmaceutical contents.

Protection against critical cross-contamination

The same smart camera also identifies unwanted product splashing in the vial top during the same operation. Other contaminations, defects or inclusions in the container glass also become visible as darker or completely opaque objects in the transmitted light process. If necessary, the detection unit can be expanded to include an additional camera for complete coverage. This helps to prevent critical cross-contamination, particularly in the case of vials that contain two different drug components, such as a lyophilised preparation and a liquefying agent, in two different chambers.
The different container areas of such two-chamber vials are also separated from each other by a rubber stopper, the presence and position of which is checked by the module. In addition to those affected by splashing and fogging, those during which this separator is missing are also identified and removed from the production flow with the help of the electronically operated rejection system. This ensures that the two product components do not mix until it is absolutely necessary – namely, only directly before the parenteral administration of the often highly potent combination preparation.
Particular design for hygienically sensitive areas
For safe use under clean room class A conditions, the design of the detection unit ensures that no components protrude over the belt on which the injection bottles, which are not yet finally sealed, are transported. Nothing can fall into them or disturb or interrupt the laminar flow; the contents are effectively protected from contamination during the optical inspection.
The stopper fit and the unwanted presence of product splashing and product fogging are not only checked randomly, but consistently on each individual vial. The risk of leaking or contaminated injection bottles being omitted and neither inspected nor rejected during the process if necessary is practically excluded. The consistent monitoring and tracking which the comprehensive HEUFT SPECTRUM II device platform carries out contributes to this above all.
Prevention of packaging and drug waste
Due to the hygienically optimised design this is also possible under laminar flow conditions in a precisely defined class A clean room environment. Leaky, sealed, contaminated injection bottles which could potentially be affected by cross-contamination are detected under clean room conditions and withdrawn from circulation in good time before they can reach the patient.
The special adapted generation, processing and intelligent analysis of the images realises a high detection rate with a low false alarm rate. The CCI and thus the necessary drug safety is just as guaranteed as the effective protection against unnecessary packaging and drug waste!
From foreign particles in the lyophilised cake to bent pre-filled syringe needles: find out for yourself at PharmaTechnica at PharmaCongress 2026 in Wiesbaden at stand A37 what else HEUFT's intelligent detection technologies have to offer when it comes to 100% complete inspection and continuous CCIT of supposedly difficult-to-inspect products (DIP)!